CE Certification Nucleic Acid Test Factories – COVID-19 test kit (colloidal gold)-25tests/kit – CILIANG
CE Certification Nucleic Acid Test Factories – COVID-19 test kit (colloidal gold)-25tests/kit – CILIANG Detail:
Please flow the instruction leaflet carefully
INTENDED USE
Rapid SARS-CoV-2 Anigen Tet Card is n immunochromatography based one step in vitro test. it is designed for the rapaid qualitative determination of SARS-cOv-2 virus antigen in anterior nasal swabs from individuals suspected of COVID-19 within the seven days of symptom onset. Rapid SARS-Cov-2 antigen Test Card shall not be used as sole basis to diagnose or exclude SARS-CoV-2 infection.Children under 14years of age should be assister by an aduit.
SUMMARY
The novel coronaviruses belong to the B ‘genus.COVID-19 is an acute respiratory infectious disease.People are generally susceptible.Currently,the atients infected by the novel coronavirus are the main source of infection,asymptomatic infected people can also be an infectious source.Based on the current epidemiological investigation,the incubation period is 1 to 14 days,mostly 3 to 7 days.The main manifestations include fever,fatigue and dry cough.
Nasal congestion,runny nose,sore throat, myalgia and diarrhea are found in a few cases.
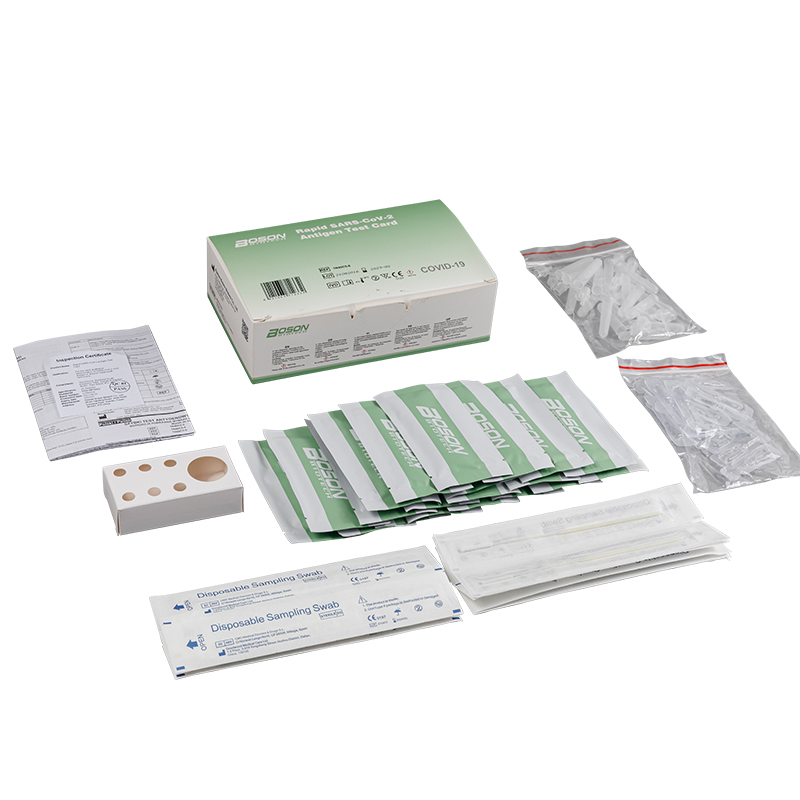
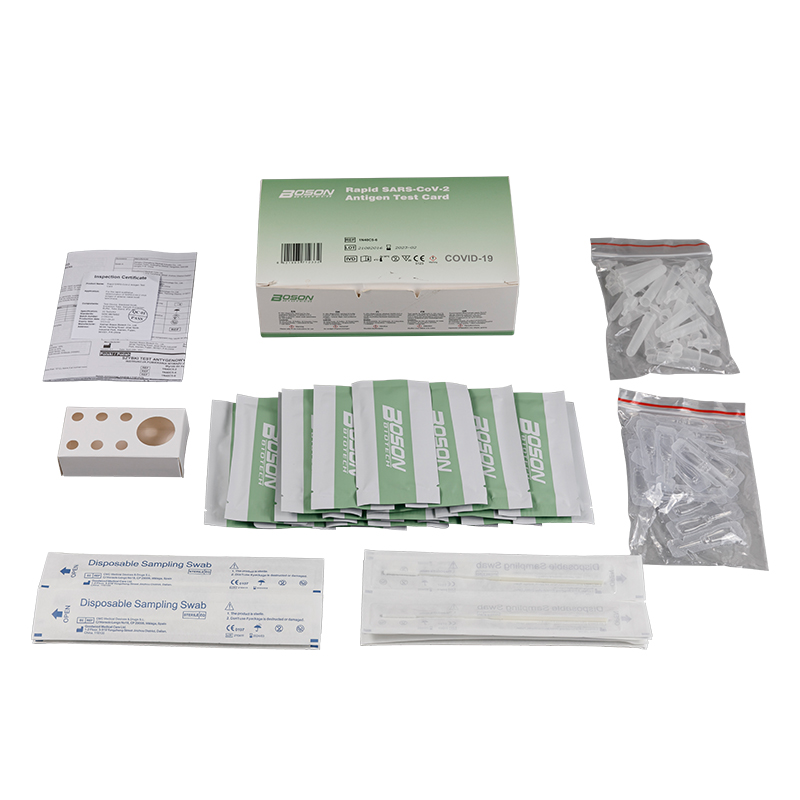
MATERIALS PROVIDED
| Components | For1 TestBox | For 5 Tess/Box | For 20 Tests/Box |
| Rapid SARS-COV-2 Antigen Test Cand (sealed fa pouch) | 1 | 5 | 20 |
| Slerile swab | 1 | 5 | 20 |
| Edracian tube | 1 | 5 | 20 |
| Sample extraction bufler | 1 | 5 | 20 |
| Instucians for use (is eafed) | 1 | 1 | 1 |
| Tube stand | 1 (packaging) | 1 | 1 |
| Sens itivity | 98.77% |
| Specificity | 99,20% |
| Accuracy | 98,72% |
A feasibility study demonstrated that:
- 99,10% of non-professionals carried out the test without requiring assistance
- 97,87% of the different types of results were interpreted correctly
INTERFERENCES
None of the following substances at the tested concentration showed any interference with the test.
Whole Blood:1%
Alkalol:10%
Mucin:2%
Phenylephrine:15%
Tobramycin:0,0004%
Oxymetazoline:15%
Cromolyn:15%
Benzocaine:0,15%
Menthol:0,15%
Mupirocin:0,25%
Zicam Nasal Spray:5%
Fluticasone Propionate:5%
Oseltamivir Phosphate:0,5%
sodium chloride:5%
Human Anti-mouse Antibody (HAMA):
60 ng/mL
Biotin:1200 ng/mL
IMPORANT INFORMATION BEFORE THE EXECUTION
1.Read this instruction guide carefully.
2. Do not use the product beyond the expiration date.
3.Do not use the product if the pouch is damaged or the seal is broken.
4. Store the test device at 4 to 30°C in the original sealed pouch. Do Not Freeze.
5.The product should be used at room temperature (15°C to 30°C). If the product has been stored in a cool area (less than 15°C), leave it at normal room temperature for 30 minutes before using.
6.Handle all specimens as potentially infectious.
7.Inadequate or inappropriate specimen collection, storage, and transport may yield inaccurate test results.
8. Use the swabs included in the test kit to ensure optimal performance of the test.
9. Correct specimen collection is the most important step in the procedure. Make sure to collect enough specimen material (nasal secretion) with the swab, especially for anterior nasal sampling.
10. Blow the nose several times before collecting specimen.
11. The specimens should be tested as soon as possible after collection.
12. Apply the drops of test specimen only to the specimen well (S).
13. Too many or too few drops of extraction solution can lead to an invalid or incorrect test result.
14. When used as intended, there should not be any contact with the extraction buffer. In case of contact with skin, eyes, mouth or other parts, rinse with clear water. If an irritation persists, consult a medical professional.
15. Children under 14 years of age should be assisted by an adult.

Product detail pictures:


Related Product Guide:
We believe in: Innovation is our soul and spirit. Top quality is our life. Purchaser need is our God for CE Certification Nucleic Acid Test Factories – COVID-19 test kit (colloidal gold)-25tests/kit – CILIANG, The product will supply to all over the world, such as: Jakarta, Lyon, Costa rica, As an experienced factory we also accept customized order and make it same as your picture or sample specifying specification and customer design packing. The main goal of the company is to live a satisfactory memory to all the customers, and establish a long term win-win business relationship. For more information, please contact us. And It is our great pleasure if you like to have a personally meeting in our office.
It can be said that this is a best producer we encountered in China in this industry, we feel lucky to work with so excellent manufacturer.