CE Certification Realtime PCR Manufacturer – COVID-19 Antigen Rapid Test Kit (Colloidal Gold) – CILIANG
CE Certification Realtime PCR Manufacturer – COVID-19 Antigen Rapid Test Kit (Colloidal Gold) – CILIANG Detail:
Limitations
1.This reagent is only used for in vitro diagnosis.
2.This reagent is only used to detect human human nasal swabs/ oropharyngeal swabs specimen. The results of other specimens may be wrong.
3.This reagent is only used for qualitative detection and can’t detect the level of novel corona virus antigen in the specimen.
4.This reagent is only a clinical auxiliary diagnostic tool. If the result is positive, it is recommended to use other methods for further examination in time and the doctor’s diagnosis shall prevail.
5.If the test result is negative and clinical symptoms persist. It is recommended to repeat sampling or use other testing methods for testing. A negative result cannot preclude the possibility of exposure to or infection with SARS-CoV-2 virus at any time.
6.The test results of the test kits are for clinicians’ reference only, and should not be used as the only basis for clinical diagnosis. The clinical management of patients should be comprehensively considered in combination with their symptoms/signs, medical history, other laboratory tests and treatment responses, etc.
7.Due to the limitation of the detection reagent methodology, the limit of detection of this reagent is generally lower than that of nucleic acid reagents. Therefore, the test personnel should pay more attention to the negative results and need to combine other test results to make a comprehensive judgment. It is recommended to use nucleic acid testing or virus isolation and culture identification methods to review negative results which have doubts.
8.Positive test results do not exclude co-infection with other pathogens.
9.False negative results may occur when the SARS-CoV-2 antigen level in the sample is lower than the detection limit of the kit or the specimen collection and transportation are not appropriate. Therefore, even if the test results are negative, the possibility of SARS-CoV-2 infection can’t be ruled out.
10.Positive and negative predictive values are highly dependent on prevalence rates. Positive test results are more likely to represent false positive results during periods of little/no SARS-CoV-2 activity when disease prevalence is low. False negative test results are more likely when prevalence of disease caused by SARS-CoV-2 is high.
11.Analysis of the possibility of false negative results:
(1)Unreasonable specimen collection, transportation and processing, low virus titer in the sample, no fresh sample or freezing and thawing cycling of the sample may lead to false negative results.
(2)The mutation of viral gene may lead to changes in antigenic determinants, which lead to negative results.
(3)The research on the SARS-CoV-2 has not been completely thorough; the virus may mutate and cause the differences for best sampling time (virus titer peak) and sampling location. Therefore, for the same patient, we can collect samples from multiple locations or follow up for multiple times reduce the possibility of false negative results.
12.Monoclonal antibodies may fail to detect, or detect with less sensitivity, SARS-CoV-2 viruses that have undergone minor amino acid changes in the target epitope region.

Product detail pictures:


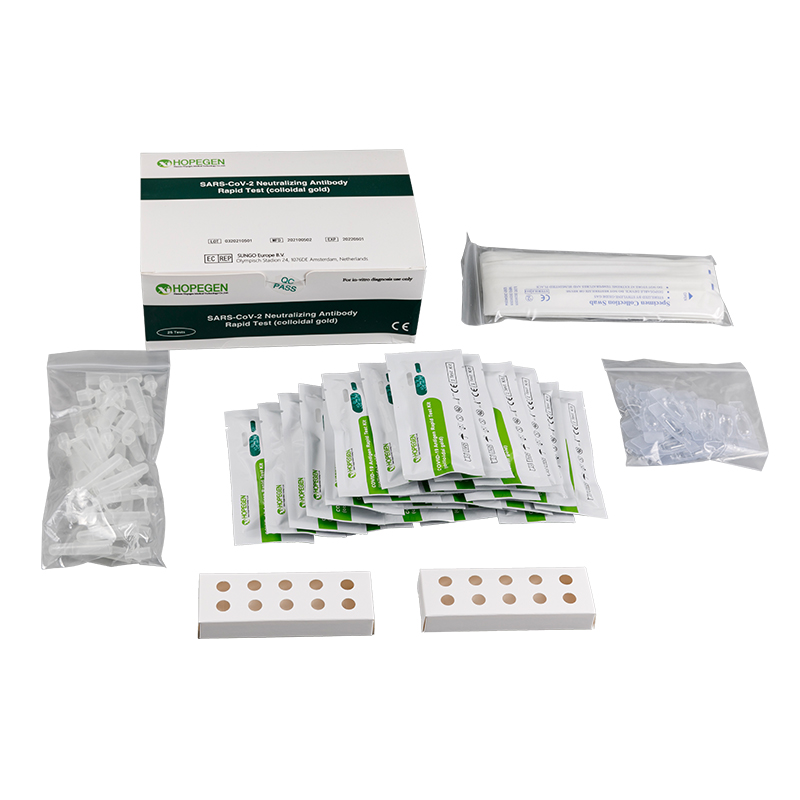
Related Product Guide:
Our aim is usually to give superior quality items at aggressive rates, and top-notch company to clients around the earth. We have been ISO9001, CE, and GS certified and strictly adhere to their good quality specifications for CE Certification Realtime PCR Manufacturer – COVID-19 Antigen Rapid Test Kit (Colloidal Gold) – CILIANG, The product will supply to all over the world, such as: Nairobi, Croatia, Belarus, We aspire to meet the demands of our customers globally. Our range of merchandise and services is continuously expanding to meet customers' requirements. We welcome new and old customers from all walks of life to contact us for future business relationships and achieving mutual success!
Products and services are very good, our leader is very satisfied with this procurement, it is better than we expected,