-

CE Certification Medical Mask Equipment Manufacturer – COVID-19 Antigen Rapid Test Kit – CILIANG
Intended use COVID-19 Antigen Rapid Test Kit (Colloidal Gold) is used for in vitro qualitative detection of the SARS-CoV-2 antigen (Nucleocapsid protein) in human nasal swabs/ oropharyngeal swabs specimen. The novel corona virus belongs to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel corona virus are the main source of infection; asymptomatic infected people can also be an infectious source. B... -
CE Certification Health & Medicine Suppliers – SARS-CoV-2 Neutralizing Antibody Rapid Test(colloidal gold)-1test/kit – CILIANG
process For fingertip whole blood samples a).Clean the puncture site with the alcohol pad b).After the alcohol is dried, the fingertips are punctured with safety Lancet to form blood droplets c).The operator uses a disposable pipette to absorb 60 µL of the Fingertip whole blood sample,add it to the sample hole. Immediately add 1 drop of whole blood buffer to the sample hole 4.The test results should be read within 15 minutes. Any results read after 20 minutes are invalid. -
OEM PCR Test Factories – SARS-CoV-2 Neutralizing Antibody Rapid Test (colloidal gold) – CILIANG
test method For Venous Whole blood samples: The operator uses a disposable dropper to absorb 50ul whole blood sample, drop it into the sample hole on the test card, and immediately add 1 drop of whole blood buffer to the sample hole. Negative result if there is only a quality control line C, the detection line is colorless, indicating that SARS-CoV-2 antigen has not been detected and the result is negative. Negative result indicates that the content of the SARS-CoV-2 antigen in the sample is... -
CE Certification Virus Collection Tube Supplier – COVID-19 Antigen Rapid Test Kit (Colloidal Gold) – CILIANG
Limitations 1.This reagent is only used for in vitro diagnosis. 2.This reagent is only used to detect human human nasal swabs/ oropharyngeal swabs specimen. The results of other specimens may be wrong. 3.This reagent is only used for qualitative detection and can’t detect the level of novel corona virus antigen in the specimen. 4.This reagent is only a clinical auxiliary diagnostic tool. If the result is positive, it is recommended to use other methods for further examination in time and the ... -

OEM Benificiation Recovery Equipment Suppliers – COVID-19 Antigen test kit (colloidal gold)-1test/kit – CILIANG
Bitte befolgen Sie die Gebrauchsanweisung. VERWENDUNGSZWECK Der SARS-CoV-2 Antigen Schnelltest ist ein auf Immunchromatographie basierender, einstufiger In-vitro-Test. Er ist for die schnelle quaitative Bestimmung von SARS-COV-2-Virus-Antigen in anterioren Nas enabstrichen (Nase vorne) von Personen mit Verdacht auf COVID-19 innerhalb der ersten sieben Tage nach Aufreten der Symptome konzipiert. Der SARS-CoV-2-Antigen-Schnelltest soll nicht als einzige Grundlage for die Dlagnose oder den Aussc... -
OEM Medical HIV Testing Equipment Factory – COVID-19 Antigen Rapid Test Kit – CILIANG
Intended use COVID-19 Antigen Rapid Test Kit (Colloidal Gold) is used for in vitro qualitative detection of the SARS-CoV-2 antigen (Nucleocapsid protein) in human nasal swabs/ oropharyngeal swabs specimen. The novel corona virus belongs to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel corona virus are the main source of infection; asymptomatic infected people can also be an infectious source. B... -

OEM Neutralizing Antibody Factory – COVID-19 Antigen Rapid Test Kit – CILIANG
Intended use COVID-19 Antigen Rapid Test Kit (Colloidal Gold) is used for in vitro qualitative detection of the SARS-CoV-2 antigen (Nucleocapsid protein) in human nasal swabs/ oropharyngeal swabs specimen. The novel corona virus belongs to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel corona virus are the main source of infection; asymptomatic infected people can also be an infectious source. B... -
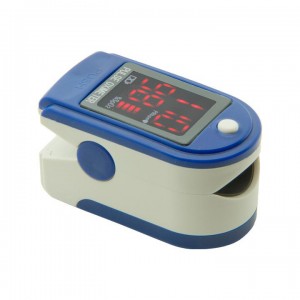
Medical Consumables Factories – Smart Ear Pulse Blood Oximeter – CILIANG
Introduction Principle of the CMS50DL Pulse Oximeter is as follows: Photoelectric Oxyhemoglobin Inspection Technology is adopted in accordance with Capacity Pulse Scanning & Recording Technology,the Pulse Oximeter can be used in measuring the pulse oxygen saturation and pulse rate through finger.The product is suitable for being used in family, hospital, oxygen bar, community healthcare, physical care in sports (It can be used before or after doing sports, and it is not recommended to use... -

China Wholesale Dental Consumables Manufacturer – Digital Pulse Oximeter For Kids WIth ISO13485 – CILIANG
Introduction CMS50M Pulse Oximeter is a non-invasive device intended for the spot-check of oxygen saturation of arterial hemoglobin(SpO2) and the pulse rate of adult and pediatric patients in home and hospital environments(including clinical use in internist/surgery, anesthesia, intensive care ect.). This device is not intended for continuous monitoring. Main Features ■Can measure SpO2 and Pulse Rate accurately ■SpO2 and Pulse Rate display,bar graph display ■Battery voltage low indication -

OEM Urine Chemistry Analyzer Suppliers – COVID-19 test kit (colloidal gold)-25tests/kit – CILIANG
Please flow the instruction leaflet carefully INTENDED USE Rapid SARS-CoV-2 Anigen Tet Card is n immunochromatography based one step in vitro test. it is designed for the rapaid qualitative determination of SARS-cOv-2 virus antigen in anterior nasal swabs from individuals suspected of COVID-19 within the seven days of symptom onset. Rapid SARS-Cov-2 antigen Test Card shall not be used as sole basis to diagnose or exclude SARS-CoV-2 infection.Children under 14years of age should be assister ... -

OEM Nucleic Acid Extraction System Supplier – COVID-19 Antigen test kit (colloidal gold)-1test/kit – CILIANG
Bitte befolgen Sie die Gebrauchsanweisung. VERWENDUNGSZWECK Der SARS-CoV-2 Antigen Schnelltest ist ein auf Immunchromatographie basierender, einstufiger In-vitro-Test. Er ist for die schnelle quaitative Bestimmung von SARS-COV-2-Virus-Antigen in anterioren Nas enabstrichen (Nase vorne) von Personen mit Verdacht auf COVID-19 innerhalb der ersten sieben Tage nach Aufreten der Symptome konzipiert. Der SARS-CoV-2-Antigen-Schnelltest soll nicht als einzige Grundlage for die Dlagnose oder den Aussc... -

CE Certification Labeling Machine Suppliers – COVID-19 Antigen Rapid Test Kit – CILIANG
Intended use COVID-19 Antigen Rapid Test Kit (Colloidal Gold) is used for in vitro qualitative detection of the SARS-CoV-2 antigen (Nucleocapsid protein) in human nasal swabs/ oropharyngeal swabs specimen. The novel corona virus belongs to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel corona virus are the main source of infection; asymptomatic infected people can also be an infectious source. B...









